Class Action Lawsuit Against Biogen, Inc. (NASDAQ:BIIB)

A class action lawsuit has been filed against Biogen Inc. (NASDAQ: BIIB) on May 22, 2024. The plaintiffs (shareholders) alleged that they purchased BIIB shares at artificially inflated prices between February 3, 2022 and February 13, 2024 (the lawsuit period) and are now seeking compensation for their financial losses. Investors who purchased Biogen shares during this period can click here to learn more about the opportunity to join the lawsuit.
Biogen is a biotechnology company focused on developing treatments for neurology, neuropsychiatry, specialized immunology and rare diseases. In particular, the company has a robust portfolio of medicines to treat multiple sclerosis (MS), spinal muscular atrophy and a defining pathology of Alzheimer’s disease (AD). Biogen and Eisai Co. Ltd. have jointly launched Leqembi, an FDA-approved drug for AD. The two companies originally planned to treat 10,000 patients with Leqembi by the end of March 2024.
Biogen’s misleading claims
Plaintiffs allege that during the Class Period, Biogen and three of its current and/or former executive officers (Individual Defendants) deceived investors by repeatedly making false and misleading public statements about the Company’s business prospects (primarily with respect to the AD portfolio) and compliance policies.
According to the complaint, the company mentioned in one of the press releases that it presented the data from the ADUHELM Phase 3 clinical trials at the annual CTAD conference. Biogen said that the studies “demonstrated a statistically significant correlation between reductions in plasma p-tau and less cognitive and functional decline in (AD).” In addition, Biogen noted in an SEC filing that it had adequate compliance controls, policies and procedures in place in the various countries in which it operated. Nevertheless, there was a possibility that company employees or agents could violate laws or regulations.
In addition, Biogen stated in a 2023 compliance report that it has established and maintained a compliance program. The company added that this program is maintained in accordance with industry laws and OIG (Office of the Inspector General of the US Department of Health and Human Services) guidelines.
This is how the truth came to light
The lawsuit alleges that the defendants failed to disclose truthful information in SEC filings and similar materials about the adequacy of certain controls and procedures, the strength of the company’s AD portfolio, and other matters. It alleges that the defendants caused Biogen’s stock to trade at artificially inflated prices during the period in question.
The information became apparent through a series of events between November 8, 2023, and February 14, 2024. These events included announcements of disappointing financial results for the third quarter, fourth quarter, and full fiscal year 2024. In addition, the lawsuit pointed to public statements about difficulties in bringing the Alzheimer’s drug Leqembi to market.
Finally, on February 14, Biogen announced that it had received a subpoena from the Department of Justice requesting information about its business activities in several countries. The company stated that it was providing the SEC with such information.
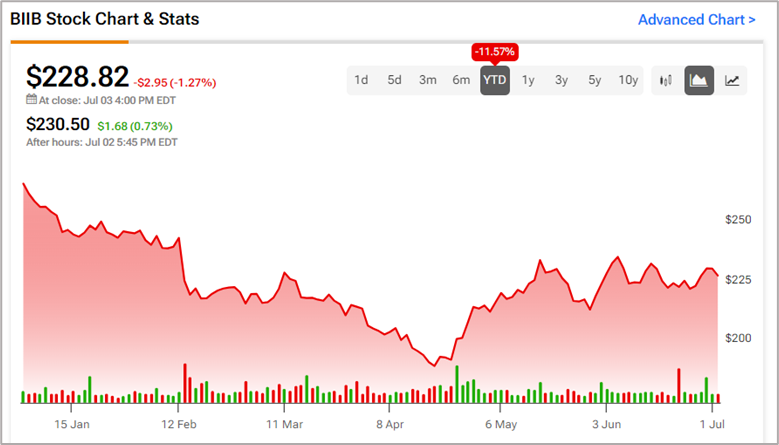
Since the beginning of the year, Biogen’s share price has fallen by almost 12%, resulting in massive losses in shareholder returns.
Disclaimer



