From waste to valuable material: The right electrolytes can improve glycerol oxidation

When biomass is converted into biodiesel, huge amounts of glycerin are produced as a byproduct. However, this byproduct has hardly been used so far, even though it could be processed into more valuable chemicals through oxidation in photoelectrochemical reactors. The reason for this: low efficiency and selectivity. A team led by Dr. Marco Favaro from the Institute for Solar Fuels at the HZB has now investigated the influence of electrolytes on the efficiency of the glycerin oxidation reaction. The results can help to develop more efficient and environmentally friendly production processes.
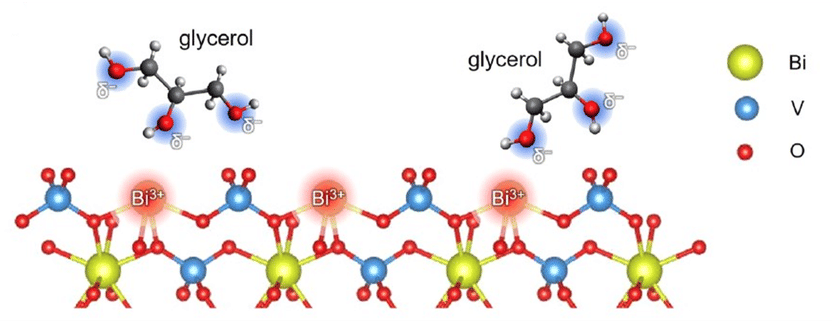
The hydroxyl groups of the glycerol are attracted to the Bi3+ ions on the surface of the BiVO4 photoanode. The electrolyte plays a crucial role in mediating these interactions.
© HZB
In 2023, around 16 billion liters of biodiesel and HVO diesel were produced in the European Union, based on corn, rapeseed or partly on residues from agricultural production. A byproduct of biodiesel production is glycerin, which can be used as a building block to produce valuable chemicals such as dihydroxyacetone, formic acid, glyceraldehyde and glycolaldehyde via a glycerin oxidation reaction (GOR). Glycerin can be oxidized electrochemically in (photo)electrochemical (PEC) reactors, which are currently being developed especially for the production of green hydrogen. However, this route in PEC plants is currently hardly used, although it could significantly increase the economic efficiency of the PEC Power-to-X process, since the oxidation of glycerin requires much less energy than hydrogen production by water splitting, but at the same time produces more valuable chemicals.
Investigate the influence of different electrolytes
Numerous studies have already examined the role of photocatalysts in PEC electrolyzers, but the role of the electrolyte has not yet been systematically analyzed. A team led by Dr. Marco Favaro at the Institute for Solar Fuels has now revealed the influence of the electrolyte composition on the efficiency and stability of glycerol oxidation.
They used a PEC cell with photoanodes made of nanoporous bismuth vanadate (BiVO4). They tested acidic electrolytes (pH = 2) with various cations and anions, including sodium nitrate (NaNO3), sodium perchlorate (NaClO4), sodium sulfate (Na2SO4), potassium sulfate (K2SO4) and potassium phosphate (KPI). “Our results showed that BiVO4 Photoanodes achieve the best performance in NaNO3 and exceed the commonly used Na2SO4 in terms of photocurrent, stability and production rates of high-quality glycerol oxidation reaction products,” summarizes Favaro.
Sodium nitrate is most effective
The team also investigated the reasons for this difference in performance: their hypothesis is that the size of the ions, their different salting-in and salting-out abilities (Hofmeister series) and their different pH buffering capacity play a role. “The composition of the electrolyte has a surprisingly significant influence on the glycerol oxidation efficiency, and we observed this trend in both bismuth vanadate and polycrystalline platinum anodes,” says PhD student Heejung Kong. This supports the assumption that these findings could be generally applicable to different materials and processes.
The choice of electrolyte is therefore of great importance for the efficiency and stability of glycerol oxidation. “Our research could help to convert biomass byproducts into valuable chemicals more efficiently and to produce valuable chemicals from waste materials while minimizing the impact on the environment,” says Favaro.


