Class Action Lawsuit Against Altimmune, Inc. (NASDAQ:ALT)

A class action lawsuit has been filed against Altimmune, Inc. (NASDAQ:ALT) on May 6, 2024. The plaintiffs (shareholders) alleged that they purchased ALT shares at artificially inflated prices between December 1, 2023 and April 26, 2024 (the lawsuit period) and are now seeking compensation for their financial losses. Investors who purchased Altimmune shares during this period can click here to learn more about participating in the lawsuit.
Altimmune is a clinical-stage biopharmaceutical company focused on discovering and developing peptide-based therapies to treat obesity and metabolic dysfunction-associated steatohepatitis (MASH). Its lead candidate is pemvidutide, a GLP-1/glucagon dual receptor agonist that helps lower blood sugar levels and promote weight loss. Altimmune’s claims that pemvidutide is a potential competitor to other GLP-1 weight loss drugs are at the heart of the lawsuit.
Misleading claims by Altimmune
Plaintiffs allege that Altimmune and three of its executive officers (Individual Defendants) deceived investors by repeatedly making false and misleading public statements about the Company’s business prospects during the Class Period.
According to the complaint, the company stated in a press release that the MOMENTUM clinical trial showed positive results in weight loss after 48 weeks of taking pemvidutide at different doses. In addition, the CEO stated in a press release that the effects of the 2.4 mg dose on weight loss in patients were significant. He also said, “For example, 48% of subjects who received the 2.4 mg dose and were obese at baseline were no longer obese at the end of the 48-week study…”
In addition, the CEO concluded that the extent of weight loss, as well as the reduction in triglyceride and LDL cholesterol levels and blood pressure shown by the study, in addition to the safety profile, are good reasons to believe that pemvidutide is different from other incretin-based therapies.
This is how the truth came to light
The complaint alleges that the defendants failed to disclose truthful information in SEC filings and related materials about the significance of the MOMENTUM litigation and related issues, and that the defendants caused Altimmune stock to trade at artificially inflated prices during the period in question.
The information became clear from events on February 13, 2024 and April 29, 2024. On February 13, short seller Kerrisdale Capital released a report saying that further investigation into Altimmune’s testing data showed that the values were not good enough to compete with approved established products or other GLP-1 agonists in clinical trials.
The 15.6% weight loss promised by pemvidutide was lower than that of other drugs such as semaglutide (Ozempic) and tirzepatide (Mounjaro). Additionally, the other drugs had the potential to control blood sugar, which pemvidutide did not. The news sent ALT shares down 18.6%.
Later, on April 29, Bloomberg The report mentioned that analyst Seamus Fernandez of research firm Guggenheim Securities downgraded his rating on ALT shares from “buy” to “hold,” causing the stock to fall nearly 12%. The firm cited the increasing likelihood of Altimmune’s lead candidate, pemvidutide, entering into a partnership.
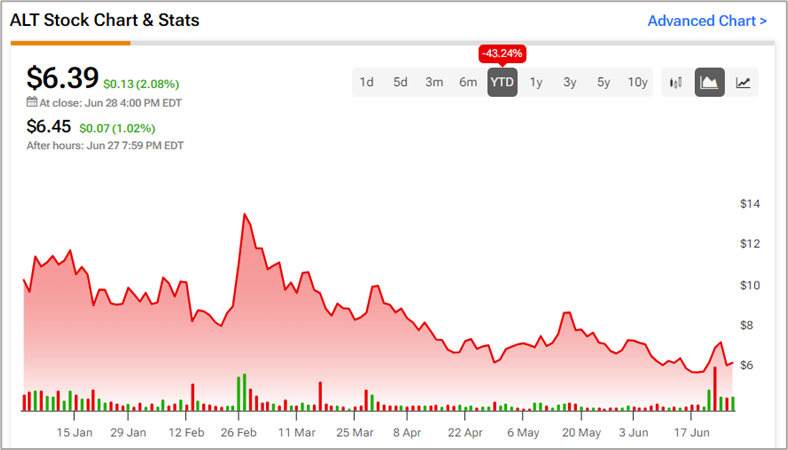
Since the beginning of the year, Altimmune shares have lost over 43%, resulting in massive losses in shareholder returns.
Disclaimer



